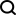Typically, when the FDA asks a pharmaceutical company to stop distributing a drug, the firm follows through.
But last week, in an unprecedented clash, Sarepta Therapeutics defied the FDA’s request to halt distribution of Elevidys, its gene therapy for Duchenne muscular dystrophy—a devastating muscle-wasting condition. However, in a surprising turnaround, Sarepta reversed course on Monday, announcing it would pause all shipments of Elevidys by Tuesday evening. This latest development came after Children’s Hospital Los Angeles decided to suspend use of the treatment amid ongoing regulatory concerns.
Elevidys is a one-time intravenous gene therapy designed for children with Duchenne muscular dystrophy, a severe form of the disease that often leads to death before age 30. It was initially approved in 2023 in a controversial move led by Peter Marks, a senior FDA official, despite reservations from agency scientists.
On Friday evening, the FDA asked Sarepta, based in Cambridge, Massachusetts, to voluntarily halt all distribution of Elevidys, following reports of three patient deaths linked to liver failure—one from Elevidys and two from a similar experimental therapy in early trials. Later that night, Sarepta pushed back, saying it would continue shipping the drug to younger, ambulatory patients (who are typically healthier), asserting that its internal review showed no new safety risks in this group. The company had already stopped supplying the drug to non-ambulatory patients.
This rare public standoff between a biotech firm and the FDA unfolds as gene therapy investment slows and Sarepta undergoes major restructuring, including hundreds of layoffs. Since last Thursday, the company’s stock has dropped nearly 40%.
The duration of the distribution pause remains uncertain. Sarepta is currently developing a stronger immune-suppression protocol that may reduce liver toxicity. However, a senior FDA official told Stat that the drug faces an “arduous and treacherous path” to regaining full market access.
Regardless of the outcome, this ongoing controversy underscores a deeper ethical dilemma: how to weigh the risks of experimental therapies against the grim prognosis of fatal childhood diseases, and where to draw the line between hope and harm.
Why Moderna Is Turning to Quantum Computing

Messenger RNA (mRNA) technology holds immense potential for next-generation vaccines and treatments, as proven during the pandemic. Its key advantage lies in instructing the body to produce therapeutic proteins itself, rather than introducing them directly.
Yet, designing effective mRNA medicines remains a major hurdle, according to Wade Davis, Moderna’s vice president of computational science. The challenge stems from mRNA’s inherent instability—it’s naturally designed to be a temporary blueprint for cells, so it degrades quickly. For medical use, scientists must engineer it to last long enough to deliver a therapeutic dose, but not so long that it causes side effects.
The complexity is staggering. Davis explained that there are 10^623 possible nucleotide combinations that could encode the same protein as Moderna’s COVID-19 vaccine. “There are only 10^80 particles in the universe,” he noted. “The scale is incomprehensible.”
Traditional computers—even supercomputers using advanced AI—struggle with such massive combinatorial problems. There’s limited structural data on mRNA to train AI models, and the optimization space is too vast for classical systems to explore efficiently. As a result, researchers often rely on simplified rules and approximations.
Moderna’s solution? Quantum computing. The company has partnered with IBM to leverage quantum hardware for optimizing mRNA sequences. Unlike classical computers, which process options sequentially, quantum computers can evaluate multiple possibilities simultaneously—making them ideal for large-scale optimization.
Using IBM’s quantum systems, Moderna successfully predicted a 60-nucleotide mRNA sequence, shattering the previous record of eight. While this is still far from the thousands of nucleotides needed for real-world therapies, it marks a critical proof of concept. Davis believes quantum algorithms open “a fundamentally different path” through the vast landscape of molecular possibilities—potentially uncovering breakthroughs unreachable through conventional methods.
BIOTECH AND PHARMA
CAR-T cell therapy has transformed treatment for blood cancers like leukemia and lymphoma, sending many patients into long-term remission. But it has largely failed against solid tumors, which account for the majority of cancer cases. Dispatch Bio aims to change that. The three-year-old biotech emerged from stealth today with $216 million in funding, led by Bob Nelsen’s ARCH Venture Partners and the Parker Institute for Cancer Immunotherapy.
One major obstacle in treating solid tumors is that CAR-T cells struggle to identify and attack cancer cells without harming healthy tissue. Dispatch Bio’s CEO, Sabah Oney, explained to Forbes that their approach uses a specially engineered viral vector that infects tumor cells and “tags” them with a protein marker, making them visible to T-cells. The virus replicates using the cancer cell’s own machinery, making resistance less likely. Because healthy cells don’t support this replication, the risk of off-target effects is reduced.
So far, the Philadelphia-based company has tested its platform in human cells and animal models. Clinical trials are slated to begin in 2026, though the initial disease target has not yet been finalized. “Our goal is to scale this platform to reach millions of patients,” Oney said. “We’re talking about lung, breast, colon—potentially all major solid tumors—with one foundational therapy.”
Plus: AstraZeneca plans to invest $50 billion in U.S.-based drug development and manufacturing by 2030, as pharmaceutical companies brace for potential tariffs under a future Trump administration.
DIGITAL HEALTH AND AI
Mental health tech startup Slingshot AI has launched Ash, an AI-powered mental health chatbot designed to support individuals managing anxiety, depression, and emotional distress. The company also announced a $53 million funding round led by Forerunner Ventures and Radical Ventures, bringing its total capital to $93 million at an undisclosed valuation. Ash has already been beta-tested with over 50,000 users.
Plus: Aidoc, a company offering FDA-cleared AI tools for radiologists, secured $150 million in new funding from General Catalyst and Square Peg, also at an undisclosed valuation.
PUBLIC HEALTH AND HOSPITALS
The FDA has appointed George Tidmarsh, a pediatric oncologist and seasoned biopharma executive, as the new director of the Center for Drug Evaluation and Research (CDER). He succeeds Jacqueline Corrigan-Curay, who had served in an interim role after Patrizia Cavazzoni stepped down in January. Tidmarsh has led the development of seven FDA-approved drugs and previously served as CEO of multiple pharmaceutical firms. He is also an adjunct professor at Stanford School of Medicine. His appointment comes during a period of significant turmoil at the FDA under HHS Secretary Robert F. Kennedy Jr.
WHAT WE’RE READING
How aggressive organ donation policies have led to allegations of premature organ retrieval in some cases.
Wellness startup Truemed, co-founded by Calley Means—a close aide to RFK Jr.—enables users to purchase luxury wellness products like Peloton bikes and $9,000 saunas using pre-tax healthcare funds.
Steward Health, a now-bankrupt hospital chain, has sued its former CEO, accusing him of insider deals that siphoned assets and contributed to the system’s collapse.
Major AI companies, including OpenAI and Grok, have quietly removed disclaimers warning users that their chatbots are not medical professionals.
Omega Funds, a life sciences investment firm, has closed its eighth fund with $647 million in commitments.
Scientists have developed a novel vaccine delivery method using dental floss, which has shown success in early mouse trials.
Former Citigroup chairman Sandy Weill donated $100 million to launch an AI-driven cancer research center on the West Coast.
MORE FROM FORBES
ForbesVibe Coding Turned This Swedish AI Unicorn Into The Fastest Growing Software Startup EverBy Iain MartinForbesWhy JPMorgan Is Hitting Fintechs With Stunning New Fees For Data AccessBy Jeff KauflinForbesGo Back To The Office, But Bring Your Own Snacks. Blame Congress.By Kelly Phillips Erb
The above is the detailed content of InnovationRx: Sarepta Blinks In Showdown With FDA. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undress AI Tool
Undress images for free

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Clothoff.io
AI clothes remover

Video Face Swap
Swap faces in any video effortlessly with our completely free AI face swap tool!

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 AI Investor Stuck At A Standstill? 3 Strategic Paths To Buy, Build, Or Partner With AI Vendors
Jul 02, 2025 am 11:13 AM
AI Investor Stuck At A Standstill? 3 Strategic Paths To Buy, Build, Or Partner With AI Vendors
Jul 02, 2025 am 11:13 AM
Investing is booming, but capital alone isn’t enough. With valuations rising and distinctiveness fading, investors in AI-focused venture funds must make a key decision: Buy, build, or partner to gain an edge? Here’s how to evaluate each option—and pr
 AGI And AI Superintelligence Are Going To Sharply Hit The Human Ceiling Assumption Barrier
Jul 04, 2025 am 11:10 AM
AGI And AI Superintelligence Are Going To Sharply Hit The Human Ceiling Assumption Barrier
Jul 04, 2025 am 11:10 AM
Let’s talk about it. This analysis of an innovative AI breakthrough is part of my ongoing Forbes column coverage on the latest in AI, including identifying and explaining various impactful AI complexities (see the link here). Heading Toward AGI And
 Kimi K2: The Most Powerful Open-Source Agentic Model
Jul 12, 2025 am 09:16 AM
Kimi K2: The Most Powerful Open-Source Agentic Model
Jul 12, 2025 am 09:16 AM
Remember the flood of open-source Chinese models that disrupted the GenAI industry earlier this year? While DeepSeek took most of the headlines, Kimi K1.5 was one of the prominent names in the list. And the model was quite cool.
 Future Forecasting A Massive Intelligence Explosion On The Path From AI To AGI
Jul 02, 2025 am 11:19 AM
Future Forecasting A Massive Intelligence Explosion On The Path From AI To AGI
Jul 02, 2025 am 11:19 AM
Let’s talk about it. This analysis of an innovative AI breakthrough is part of my ongoing Forbes column coverage on the latest in AI, including identifying and explaining various impactful AI complexities (see the link here). For those readers who h
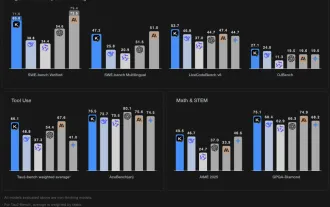
 Grok 4 vs Claude 4: Which is Better?
Jul 12, 2025 am 09:37 AM
Grok 4 vs Claude 4: Which is Better?
Jul 12, 2025 am 09:37 AM
By mid-2025, the AI “arms race” is heating up, and xAI and Anthropic have both released their flagship models, Grok 4 and Claude 4. These two models are at opposite ends of the design philosophy and deployment platform, yet they
 Chain Of Thought For Reasoning Models Might Not Work Out Long-Term
Jul 02, 2025 am 11:18 AM
Chain Of Thought For Reasoning Models Might Not Work Out Long-Term
Jul 02, 2025 am 11:18 AM
For example, if you ask a model a question like: “what does (X) person do at (X) company?” you may see a reasoning chain that looks something like this, assuming the system knows how to retrieve the necessary information:Locating details about the co
 This Startup Built A Hospital In India To Test Its AI Software
Jul 02, 2025 am 11:14 AM
This Startup Built A Hospital In India To Test Its AI Software
Jul 02, 2025 am 11:14 AM
Clinical trials are an enormous bottleneck in drug development, and Kim and Reddy thought the AI-enabled software they’d been building at Pi Health could help do them faster and cheaper by expanding the pool of potentially eligible patients. But the
 Senate Kills 10-Year State-Level AI Ban Tucked In Trump's Budget Bill
Jul 02, 2025 am 11:16 AM
Senate Kills 10-Year State-Level AI Ban Tucked In Trump's Budget Bill
Jul 02, 2025 am 11:16 AM
The Senate voted 99-1 Tuesday morning to kill the moratorium after a last-minute uproar from advocacy groups, lawmakers and tens of thousands of Americans who saw it as a dangerous overreach. They didn’t stay quiet. The Senate listened.States Keep Th