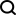FDA's New AI Tool Cuts Review Time From 3 Days To 6 Minutes
Jun 06, 2025 am 11:15 AM
FDA commissioner Dr. Marty Makary announced a significant milestone: “We’ve achieved our goal earlier than expected and within budget,” he stated. “Tasks that used to take a scientific reviewer two to three days [previously] now take only six minutes.”
What ELSA Offers… And What It Doesn’t
The FDA employs thousands of reviewers, analysts, and inspectors who handle vast amounts of unstructured data like clinical trial documents, safety reports, and inspection records. Automating even a fraction of this workload yields substantial benefits.
ELSA assists FDA teams in accelerating various critical processes. Staff are currently utilizing it to summarize adverse event data for safety evaluations, compare drug labels, create basic code for nonclinical database setup, and pinpoint high-priority sites for inspections, among other duties.
This final function—using data to determine where inspectors should focus their efforts—could influence how the FDA monitors the pharmaceutical and food supply chains and enhances the delivery of its services.
Crucially, though, the tool doesn’t operate independently without human oversight. The system organizes information so that experts can make decisions more swiftly. It streamlines routine tasks rather than handling complex judgments.
Safety Focus: No Industry Data, No External Training
One of the major concerns surrounding AI systems in the public sector involves the utilization of data and third-party AI systems. Makary tackled this issue head-on by stating that “All information remains within the agency. The AI models aren’t being trained on data provided by the industry.”
This stands in stark contrast to AI methods employed in the private sector, where numerous large language models have faced criticism for being trained on proprietary or user-generated content. In corporate environments, this has fueled a growing need for "air-gapped" AI solutions that keep data confined within the organization.
Thus, the FDA’s approach differs from many commercial tools, which frequently depend on open or external data sources. The agency isn’t developing a publicly accessible product; instead, it’s creating a tightly controlled internal system designed to improve its operations.
Other Federal Agencies Are Gradually Advancing with AI
Federal departments have been sluggish in moving beyond AI experimentation. The Department of Veterans Affairs has begun testing predictive tools to manage appointments. The SEC has explored market surveillance AI for an extended period. Yet, few have advanced to full-scale implementation.
The federal government employs thousands of workers processing immense quantities of information, much of it unstructured and stored in documents, files, and even paper. Consequently, AI is primarily being applied to operational and process-related activities. It appears poised to become a crucial component of how agencies handle data, provide recommendations, and execute actions.
Nevertheless, amidst these AI advancements, U.S. federal agencies, particularly the FDA, have experienced reductions in personnel and government contracts. The FDA has encountered notable decreases in staffing levels and programmatic cuts, affecting various aspects of its operations. These AI improvements may help mitigate some of these cuts or potentially amplify their effects on staffing and programs at the FDA.
Makary succinctly expressed that ELSA marks the start of AI integration within the FDA.
“Today’s introduction of ELSA will be the first of many initiatives to follow,” he noted. “This is how we’ll enhance our service to the American people.”
The above is the detailed content of FDA's New AI Tool Cuts Review Time From 3 Days To 6 Minutes. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undress AI Tool
Undress images for free

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Clothoff.io
AI clothes remover

Video Face Swap
Swap faces in any video effortlessly with our completely free AI face swap tool!

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 AI Investor Stuck At A Standstill? 3 Strategic Paths To Buy, Build, Or Partner With AI Vendors
Jul 02, 2025 am 11:13 AM
AI Investor Stuck At A Standstill? 3 Strategic Paths To Buy, Build, Or Partner With AI Vendors
Jul 02, 2025 am 11:13 AM
Investing is booming, but capital alone isn’t enough. With valuations rising and distinctiveness fading, investors in AI-focused venture funds must make a key decision: Buy, build, or partner to gain an edge? Here’s how to evaluate each option—and pr
 AGI And AI Superintelligence Are Going To Sharply Hit The Human Ceiling Assumption Barrier
Jul 04, 2025 am 11:10 AM
AGI And AI Superintelligence Are Going To Sharply Hit The Human Ceiling Assumption Barrier
Jul 04, 2025 am 11:10 AM
Let’s talk about it. This analysis of an innovative AI breakthrough is part of my ongoing Forbes column coverage on the latest in AI, including identifying and explaining various impactful AI complexities (see the link here). Heading Toward AGI And
 Kimi K2: The Most Powerful Open-Source Agentic Model
Jul 12, 2025 am 09:16 AM
Kimi K2: The Most Powerful Open-Source Agentic Model
Jul 12, 2025 am 09:16 AM
Remember the flood of open-source Chinese models that disrupted the GenAI industry earlier this year? While DeepSeek took most of the headlines, Kimi K1.5 was one of the prominent names in the list. And the model was quite cool.
 Future Forecasting A Massive Intelligence Explosion On The Path From AI To AGI
Jul 02, 2025 am 11:19 AM
Future Forecasting A Massive Intelligence Explosion On The Path From AI To AGI
Jul 02, 2025 am 11:19 AM
Let’s talk about it. This analysis of an innovative AI breakthrough is part of my ongoing Forbes column coverage on the latest in AI, including identifying and explaining various impactful AI complexities (see the link here). For those readers who h
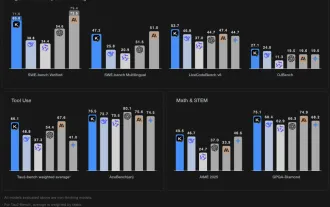
 Grok 4 vs Claude 4: Which is Better?
Jul 12, 2025 am 09:37 AM
Grok 4 vs Claude 4: Which is Better?
Jul 12, 2025 am 09:37 AM
By mid-2025, the AI “arms race” is heating up, and xAI and Anthropic have both released their flagship models, Grok 4 and Claude 4. These two models are at opposite ends of the design philosophy and deployment platform, yet they
 Chain Of Thought For Reasoning Models Might Not Work Out Long-Term
Jul 02, 2025 am 11:18 AM
Chain Of Thought For Reasoning Models Might Not Work Out Long-Term
Jul 02, 2025 am 11:18 AM
For example, if you ask a model a question like: “what does (X) person do at (X) company?” you may see a reasoning chain that looks something like this, assuming the system knows how to retrieve the necessary information:Locating details about the co
 Senate Kills 10-Year State-Level AI Ban Tucked In Trump's Budget Bill
Jul 02, 2025 am 11:16 AM
Senate Kills 10-Year State-Level AI Ban Tucked In Trump's Budget Bill
Jul 02, 2025 am 11:16 AM
The Senate voted 99-1 Tuesday morning to kill the moratorium after a last-minute uproar from advocacy groups, lawmakers and tens of thousands of Americans who saw it as a dangerous overreach. They didn’t stay quiet. The Senate listened.States Keep Th
 This Startup Built A Hospital In India To Test Its AI Software
Jul 02, 2025 am 11:14 AM
This Startup Built A Hospital In India To Test Its AI Software
Jul 02, 2025 am 11:14 AM
Clinical trials are an enormous bottleneck in drug development, and Kim and Reddy thought the AI-enabled software they’d been building at Pi Health could help do them faster and cheaper by expanding the pool of potentially eligible patients. But the